Definition: a neurotransmitter formed in pre- and postganglionic
synapses of the parasympathetic nervous system (acetylcholine).
Introduction:
The chemical identity of
neurotransmitters is often difficult to determine experimentally. For example,
it is easy using an electron microscope to recognize vesicles on the
presynaptic side of a synapse, but it may not be easy to determine directly
what chemical is packed into them. The difficulties led to many historical
controversies over whether a given chemical was or was not clearly established
as a transmitter. In an effort to give some structure to the arguments,
neurochemists worked out a set of experimentally tractable rules. According to
the prevailing beliefs of the 1960s, a chemical can be classified as a
neurotransmitter if it meets the following conditions:
- There are precursors and/or synthesis enzymes located in the presynaptic side of the synapse.
- The chemical is present in the presynaptic element.
- It is available in sufficient quantity in the presynaptic neuron to affect the postsynaptic neuron.
- There are postsynaptic receptors and the chemical is able to bind to them.
- A biochemical mechanism for inactivation is present.
Modern advances in pharmacology,
genetics, and chemical neuroanatomy have greatly reduced the importance of
these rules. A series of experiments that may have taken several years in the
1960s can now be done, with much better precision, in a few months. Thus, it is
unusual nowadays for the identification of a chemical as a neurotransmitter to
remain controversial for very long periods of time.
Types
of neurotransmitters
There are many different ways to
classify neurotransmitters. Dividing them into amino
acids, peptides, and monoamines
is sufficient for some classification purposes.
Major neurotransmitters:
- Amino acids: glutamate, aspartate, D-serine, γ-aminobutyric acid (GABA), glycine
- Monoamines and other biogenic amines: dopamine (DA), norepinephrine (noradrenaline; NE, NA), epinephrine (adrenaline), histamine, serotonin (SE, 5-HT)
- Others: acetylcholine (ACh), adenosine, anandamide, nitric oxide, etc.
In addition, over 50 neuroactive peptides have been found, and new ones are discovered regularly.
Many of these are "co-released" along with a small-molecule
transmitter, but in some cases a peptide is the primary transmitter at a
synapse. β-endorphin
is a relatively well known example of a peptide neurotransmitter; it engages in
highly specific interactions with opioid
receptors in the central nervous system.
Single ions, such as synaptically released zinc, are also considered neurotransmitters by some, as are some
gaseous molecules such as nitric oxide (NO) and carbon
monoxide (CO). These are not classical
neurotransmitters by the strictest definition, however, because although they
have all been shown experimentally to be released by presynaptic terminals in
an activity-dependent way, they are not packaged into vesicles.
By far the most prevalent
transmitter is glutamate, which is excitatory at well over 90% of the synapses
in the human brain. The next most prevalent is GABA, which is inhibitory at
more than 90% of the synapses that do not use glutamate. Even though other transmitters
are used in far fewer synapses, they may be very important functionally—the
great majority of psychoactive drugs exert their effects by altering the
actions of some neurotransmitter systems, often acting through transmitters
other than glutamate or GABA. Addictive drugs such as cocaine and amphetamine
exert their effects primarily on the dopamine system.
The addictive opiate drugs exert their effects primarily as functional analogs of
opioid peptides,
which, in turn, regulate dopamine levels.
Cholinergic Stimulating Agents:
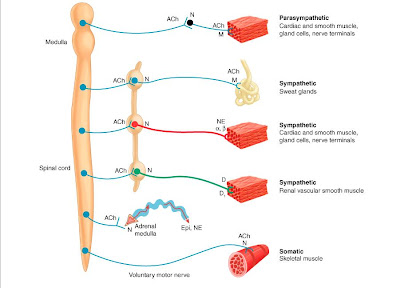
Acetylcholine is the chemical
transmitter for nerves of the parasympathetic, somatic, preganglionic
sympathetic, and parts of the central nervous system. Acetylcholine is
synthesized by the transfer of an acetyl group from acetyl CoA to choline, a
normal constituent of the diet.
Acetylcholine is concentrated in
large amounts in presynaptic vesicles, which release their contents into the
synapse when voltage-gated calcium channels open in response to membrane depolarization.
Upon interaction with the receptor,
acetylcholine produces an influx of sodium through a ligand-gated ion channel
which sends the impulse.
After acetylcholine interacts with
the cholinergic receptor it is very rapidly hydrolyzed by the enzyme
acetylcholinesterase. The hydrolysis reaction is the reverse of the synthesis
reaction except that choline and acetic acid are products. The choline is
retaken up by the nerve ending where it is reused for synthesis of new
molecules of acetylcholine.
Acetylcholine acts on two vastly
different classes of receptors - nicotinic receptors (with two subtypes, one at
the neuromuscular junction of skeletal muscle, the other within ganglia and the
CNS), and muscarinic receptors (widely distributed within both peripheral and
central nervous systems). Muscarinic receptors originally were distinguished
from nicotinic receptors by the selectivity of the agonists muscarine and
nicotine respectively. Notice the similarities in structure for all three of
these compounds.
Although
there appears to be at least two cholinergic receptor sites, they are similar
enough to be considered as one. The acetylcholine interacts with the receptor
site through ionic attraction of the positive nitrogen, polar attraction of the
ester group, and through hydrophobic interactions with the methyl groups.
Stimulation:
Stimulation of cholinergic nerves is
achieved either directly or indirectly. Direct acting agents (agonists)
activate the receptor site by mimicking the effects of acetylcholine.
Cholinesterase inhibitors act indirectly by preventing the enzyme from hydrolyzing
(inactivating) acetylcholine at the receptor site. This inhibition permits the
buildup of acetylcholine and results in more intensive and prolonged activation
of the receptor site. The effects of cholinergic stimulation include:
vasodilation of blood vessels; slower heart rate; constriction of bronchioles
and increased secretion of mucus in the respiratory tract; intestinal cramps;
secretion of salvia; sweat and tears; and constriction of eye pupils.
Direct Acting Cholinergic Agents - Agonists:
Direct acting cholinergic agents act
as agonists and initiate stimulant type responses at the receptor site. Direct
stimulation of acetylcholine receptors is achieved by: Arecholine, Pilocarpine,
Urecholine(Betanechol), Carbachol, Choline, Metacholine, Mushrooms (Boletus
sp., Clitocybe sp. , Inocybe sp.)
Drugs: Urecholine and philocarpine are direct acting drugs.
Urecholine is used to restore parasympathetic tone to smooth muscles of the
intestinal tract and bladder following abdominal surgery. Pilocarpine is used
to constrict pupils and reduce pressure caused by glaucoma. Pilocarpine
contracts the ciliary muscle with causes the iris to be withdrawn. This action
permits drainage of the aqueous humor and thus relieves the pressure due to a
glaucoma condition.
Cholinergic
Poison agents
which mimic the structure of acetylcholine include two poisons: muscarine
- an alkaloid present in poisonous mushrooms and nicotine from
cigarettes.
Muscarinic effects are those of
parasympathetic overactivity and include bradycardia, pinpoint pupils,
sweating, blurred vision, excessive lacrimation, excessive bronchial
secretions, wheezing, dyspnoea, coughing, vomiting, abdominal cramping,
diarrhea, and urinary and fecal incontinence.
Nicotine: Nicotinic effects are those of sympathetic overactivity and
neuromuscular dysfunction and include tachycardia, hypertension, dilated
pupils, muscle fasciculation and muscle weakness.
Accidental
ingestion of these poisons may produce death from heart failure unless treated
with a suitable antidote. Atropine blocks the receptor site to decrease the
stimulant effects produced by the muscarine type poisons, but has no effect on
nicotine receptors.
Nicotinic and muscarinic receptors
Neurotransmitters interact with
specific receptor molecules (proteins). In the case of acetylcholine, these are
nicotinic and muscarinic receptors - so called because they were originally
distinguished on the basis of their selectivity to nicotine and muscarine,
respectively. Activation of these receptors by released ACh continues the
process of signal transmission.
Most peripheral acetylcholine
receptors (AChR ) are nicotinic, such as those on the heart or at the
neuromuscular junction, which is why nicotinic antagonists (a drug that blocks
nicotinic receptors, such as d-tubocurarine, from the arrow poison, curare) are
used as muscle relaxants. Nicotinic agonists (nicotine mimicking drugs),
including nicotine (7), have been found to improve attention of
Alzheimer patients. Another nicotinic agonist, ABT-418 (8), displays
cognition-enhancing properties.
Scientists are currently
investigating novel nicotinic agonists which are selective for particular
nicotinic receptor subtypes. A number of such compounds are in clinical
development, many of which are less toxic than nicotine.
Muscarinic receptors are stimulated
by muscarine, and blocked by atropine, which is the poison (also known as
deadly nightshade) found in the belladonna plant. Muscarinic receptors have
been divided into five receptor subtypes. Several synthetic muscarinic agonists
have been made, based on the structure of acetylcholine. Research points to the
three-membered ring of acetoxycyclopropyl-trimethylammonium iodide having the
highest intrinsic activity; the trans-isomer (9) having much
higher activity than the cis-isomer. Such information will be
important in the development of new drugs.
References
1. A. M. Palmer and P. T. Francis
in Principles and practice of geriatric medicine, 4th edn, M. S. J.
Pathy, A. J. Sinclair and J. E. Morley (eds), pp59-67. Chichester: John Wiley,
2006.
2. A. M. Palmer, Neurodegeneration, 1995, 5 , 381.
3. A. M. Palmer, Investigational Drugs, 2003, 4 , 833.
4. N. A. Clarke and P. T. Francis, Expert Rev. Neurother., 2005, 5 , 671.
5. See Memantine website.
6. A. M. Palmer, Trends Pharmacol. Sci., 2002, 23 , 426.
2. A. M. Palmer, Neurodegeneration, 1995, 5 , 381.
3. A. M. Palmer, Investigational Drugs, 2003, 4 , 833.
4. N. A. Clarke and P. T. Francis, Expert Rev. Neurother., 2005, 5 , 671.
5. See Memantine website.
6. A. M. Palmer, Trends Pharmacol. Sci., 2002, 23 , 426.